|
pH
Control: A Magical Mystery Tour
Systems
for pH control are characterized by extreme rangeability and sensitivity,
and are also subject to difficulties arising from contact between
measuring electrodes and hostile fluids. Case histories of representative
installations show that success in implementing these control
systems depends not only on assessing the complexity of the loop
and selecting a control strategy but also on recognizing and avoiding
pitfalls while specifying and installing instrumentation, equipment,
and piping.
I
do not pretend to be the world's champion pH problem solver. But,
I have helped rescue over 50 foundering pH control systems in
the past 5 years and am still rational enough to tell you about
it. Most people hardly notice that I twitch at the mention of
hydrogen ion concentration.
WHY
IT'S A PROBLEM
Why
is pH control a problem? After all, you've got an odd but simple
scale of measurement from 0 to 14 dimensionless units, measuring
electrodes that have been around long enough to be well understood
and readily applied, and instrument vendors who must have seen
every possible application by now.
Rangeability
and Sensitivity
One
basic source of difficulty is that - as countless articles, technical
papers, and textbooks point out - the pH scale corresponds to
hydrogen ion concentrations from 100 to 10-14 moles per liter.
No other common measurement covers such a tremendous range. Another
intrinsic constraint is that measuring electrodes can respond
to changes as small as 0.001 pH, so instruments can track hydrogen
ion concentration changes as small as 5x10-10 moles per liter
at 7 pH. No other common measurement has such tremendous sensitivity.
The
implications of such great rangeability and sensitivity can be
illustrated by considering a continuous feedback neutralization
system for a strong acid and a strong base. The reagent flow should
essentially be proportional to the difference between the hydrogen
ion concentration of the process fluid and the setpoint. A reagent
control valve must therefore have a rangeability greater than
10,000,000:1 for a setpoint of 7 pH when the incoming stream fluctuates
between 0 and 7 pH. Moreover, uncertainties in the control valve
stroke translate directly into pH errors, such that hysteresis
of only 0.00005% can cause an offset of 1 pH for a 7 pH setpoint.
The
situation is like playing golf. The distance from the tee to the
green represents rangeability and the ratio of the hole diameter
to this distance is analogous to sensitivity. For an application
requiring a strong base to neutralize a strong acid or vice versa,
the tee would be about 1,000,000 yards from the green and the
hole would be about 3-1/2 inches in diameter. A hole-in-one is
impossible. And using the same size control valve at each stage
would be like hiring a gorilla to drive the ball to the green
in one stroke, then finding that it tends to overshoot the hole
on the putt.
How is it even possible to control a process under these conditions?
Rangeability and sensitivity limitations can be overcome by approaching
the setpoint in stages, using successively smaller control valves
with high performance positioners.
The
real world
A
host of other constraints adds to the difficulties of pH control.
These range from the necessity of wetting the electrodes - with
consequent susceptibility to leakage and attack by the fluid,
to long delays introduced by the need to mix large volumes of
process material with small amounts of reagent. Even with a good
understanding of measurement and control concepts, these real-world
effects introduce an element of magical mystery into pH.
|
TABLE
I.
The
Facts of Life
- Instrumentation
is frequently the source of disturbance for pH systems,
through repeatability error, measurement noise, or valve
hysteresis.
- In-line
pH loops will oscillate, regardless of controller modes
and tuning, if setpoints are on the steep parts of the
titration curves.
- pH
electrode submersion assemblies with unencapsulated terminations
below the liquid surface will eventually have wet terminations.
- Reagent
control valves that are not close-coupled to the injection
point on in-line systems will cause reagent delivery delays
large enough to describe the tools of your trade in words
your sister may not even know.
- You
need either a flowmeter or a seer to diagnose reagent
delivery problems.
- Flow
feedforward signals should be multiplied by pH controller
outputs and employed to operate reagent valves directly
or to establish reagent flow control setpoints
- Transportation
delays to pH electrodes in analyzer houses will exceed
mixing deadlines - such that increasing comfort in checking
the electrodes is offset by decreasing comfort in checking
trend recordings.
- Injection
electrodes should be preferred to sample holder assemblies
whenever possible to reduce maintenance problems and improve
response times - but not all injection electrodes are
created equal.
- Large
tanks are fine if you don't have to control them; use
the volume upstream to reduce reagent consumption or downstream
to reduce control error. If you can't make-up your mind
where to use one, put it downstream.
- Install
one or three but never two electrodes for a pH measurement.
|
SOME
TYPICAL PROBLEMS
No
pH complications are really typical. And the systems that are
easy to implement don't get referred back to those of us whom
InTech refers to as the noodnicks from Central Engineering. But
the installations I will describe are typical of those I have
encountered recently, and illustrate the types of problems that
you can expect.
To avoid arguments with our Legal Department about proprietary
information, I will not mention any places or names; I'd even
prefer that you forget my name when you finish reading. Also,
to help guide you through my tribulations, and those you will
encounter yourselves, I've prepared Table I, enumerating what
you can think of as the Facts of Life. Commit this Table to memory;
you'll be quizzed on it in the morning.
Where's
the tank?
An
application involved a strong acid waste flow, to be neutralized
by a strong basic reagent. It was called in because the pH was
swinging from 0 to 14 despite efforts to tune the controllers,
manually manipulate the reagent, and regulate the influent flow.
When I got to the plant, I gazed over the horizon and didn't see
any tanks. I suddenly realized that I had a major problem.
Figure 1a shows the original control system. This used a ratio
controller to proportion reagent to acid waste flow upstream of
an in-line mixer. A separate pH controller was used in a loop
on a sump. The system designers did not realize that the flow
measurement error and the flow control valve hysteresis must both
be less than 0.00005% to stay within 1 pH of the 7 pH setpoint.
They assumed that disturbances would be small since the change
in waste composition was slow and its flow was fixed by a controller.
The design team did not know Fact of Life #1.
A
system involving a strong acid and a strong base normally requires
three stages of control to hold a solution within 1 pH of 7 pH
(Ref 1). Since cost was stressed as a factor, I kept the existing
mixer and sump as one stage and added two vertical well-mixed
tanks downstream for the second and third stages. Further, I agreed
to not install controls on the third stage until the need was
demonstrated. The third stage volume therefore served as a filter
for the oscillation from the second stage.
For
the first stage of control, we started by replacing the ratio
flow system with a fast inline pH loop. This received a remote
setpoint from a second pH controller on the sump. The fast in-line
loop would initiate the correction and depend on the sump volume
to average out hydrogen ion concentration deviations. Linear control
system analysis predicted that this combination would be as effective
as a single well-mixed vertical tank. It didn't work. Dynamic
simulation showed that the in-line loop would oscillate between
0 and 14 pH for all controller settings. A plant test confirmed
the result.
At first, I thought the sump was somehow not providing the anticipated
filtering. Then I remembered Fact of Life #2. The filter was acting
on hydrogen ion concentration, not pH. The sump was attenuating
concentration oscillations by a factor of 100, but is corresponded
to a decrease of only 2 pH. Attenuation was improved by reducing
the distance from the mixer to the control valve and electrodes
so the oscillation was faster.
The
second state had a notch-gain pH controller was an output that
provided a pulse frequency proportional to an analog signal. Above
25% controller output, the valve was throttled normally; below
25%, valve rangeability was extended using pulse frequency or
interval control.
Figure
1b shows the upgraded installation. This system could keep pH
within the desired offset band at the outlet of the third stage.
However, the sump controller was difficult to tune and recovery
from startup or waste flow controller setpoint change was slow.
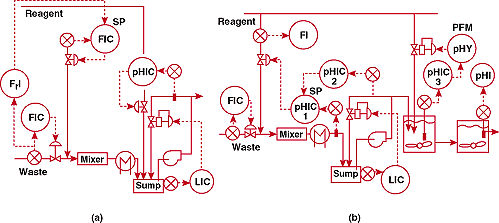
FIGURE
1. Where's the tank? (a)-unsuccessful and (b)-successful pH control
systems for a continuous neutralization process initially having
no mixing tank.
If
I were designing this system today, I would place a feedforward
loop on the sump and would install controls on the third stage.
I would also characterize the feedforward and feedback signals.
The characterization would involve calculating reagent demand
from the pH measurement using the titration curve, and using the
result as the control command. This would reduce nonlinearity,
recovery time, sensitivity, and tuning difficulty. Microprocessor-based
controllers can provide the necessary calculation accuracy and
ease of implementation.
As
with any new system, startup was not without bugs. Some were of
the common garden variety - like transposed wires and incorrectly
calibrated positioners. Others were of the magical mystery type
peculiar to pH systems.
For example, at high pH levels, the measurement went downscale
as the strong base reagent flow increased. As you can imagine,
this drove the control system - and us - kind of crazy. The difficulty
turned out to be that the measuring electrodes in the in-line
loop were not specified with high-pH glass. Normally this would
cause the measurement to read low by about 1 pH at the upper end
of the scale. In our case, it caused a reversed response. This
performance was confirmed by the vendor, and was corrected by
replacing the electrodes with low sodium ion error devices.
Another
magical mystery effect was that the electrode response for the
well mixed tank became erratic. We found water on the terminals
inside the submersion assembly. The vendor told us if we bought
an assembly that cost twice as much, the leakage would stop. We
did; it didn't. The vendor then told us to buy a newly developed
assembly, for four times the price of the original and the leakage
would surely stop. Rather than make the same mistake three times,
I shopped around and found a throwaway electrode assembly completely
encapsulated in plastic - at half the price of the original. It
worked like a charm. A similar experience with a submersion assembly
from another vendor led me to Fact of Life #3.
Where's
the valve?
Another
application required small quantities of a highly concentrated
viscous reagent for continuous neutralization of a waste stream.
The control system was so slow that disturbances passed through
the plant long before any corrective action took effect; further,
the pH trend recording had a noise band that far exceeded the
allowable setpoint offset. When I inspected the system, I stood
near the injection point at the inlet to the pipeline mixer, scanned
the horizon and didn't see any reagent control valve. I quickly
deduced I had a major problem. Figure 2a shows what I found.
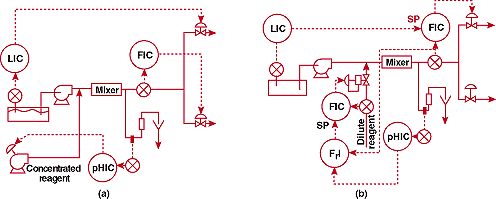
FIGURE
2. Where's the valve? (a)-unsuccessful and (b)-successful pH control
systems for a process involving a highly viscous concentrated
reagent.
Can
you spot a control problem exclusive of the pH loop in this figure?
The sump level controller sets the flow in the upper outlet branch.
The mixer flow controller simultaneously manipulates the valve
in the lower branch to keep a constant flow out of the sump. The
system is obviously overcontrolled. We got out of this mess by
cascading the level controller output to flow controller setpoint.
Now
for the pH loop. The reagent was being injected into the pipeline
under the control of a positive displacement metering pump. The
pump was about 300 feet away from the mixer. This distance caused
a delay when the pump was activated - because process fluid would
backfill the injection piping and had to be pushed out of the
line before any reagent could be delivered. It doesn't take much
fancy mathematics to figure that at one gallon per hour, it takes
an hour to push a gallon through a pipe. This led to Fact of Life
#4. We also found a delay when the speed of the pump changed,
but never really identified the cause. We would have blamed it
on air pockets, if there had been any. The answer probably lies
in the ketchup bottle - related to low flow of viscous fluids.
Anyway,
we reduced the delays and resulting noise band by an order of
magnitude when we replaced the remote metering pump with a closecoupled
control valve. The valve was manipulated using a ratio controller
to proportion the reagent flow to the sump discharge flow, correcting
the ratio with the in-line pH loop.
Some
noise still remained, due to poor distribution of the injected
reagent into the pipeline. This couldn't be eliminated, because
it required making the injection port smaller so the reagent velocity
would be larger. Unfortunately, a hole small enough to do the
job was too small to keep from plugging. The noise was more of
a nuisance on the trend chart than in the system, so the record
was cleaned up by passing the measurement signal through an electronic
filter.
We
thought our problems were over, when magical mystery reared its
ugly head. As the miniature reagent valve was stroked from closed
to open, the reagent flow measurement momentarily increased and
then went to zero. The magnetic flowmeter was immediately suspect
- but came through with a clean bill of health; we checked the
wiring and found it to be correct; the vendor examined and verified
the integrity of the electronics; we tested the meter on water
and observed that it respond correctly. We than tried changing
valve trim, but several tests yielded the same results.
I
was about to throw the tiny but costly trims away, leave the engineering
profession, and enter a seminary. During this period of contemplation,
I suddenly noticed what looked to be a reverse taper on the trims.
It was hard to tell for sure, because the parts were small, but
I confirmed the observation with a micrometer. In desperation
to get home from this startup, I calculated the contour of the
plug for a linear characteristic, made a sketch, and had the parts
machined.
The
valve worked fine with the homemade trim. The reverse taper had
caused the flow to decrease as the stroke increased. The momentary
surge inflow at the start of the stroke was caused by the plug
lifting off the seat just enough to provide a small annular clearance.
How did the reverse taper get there in the first place? I never
found out for sure, but did learn that the trims were too small
to be standard and were specially machined by the vendor for the
order. As far as I was concerned, they were too special. You can
imagine how difficult it would have been to diagnose this valve
problem if there was no reagent flow meter. This leads to Fact
of Life #5.
Another
instrumentation problem occurred later, when one of the design
engineers decided to modify the system and recover some panel
space. He installed a feedforward controller in place of the ratio
station and pH-based flow controller. The device added the flow
feedforward signal to the flow command from the pH controller.
The vendor, anxious to sell a feedforward element, thought it
was a great idea. In operation, as you should have guessed, the
flow controller readjusted its output to cancel the effect of
the feedforward signal and maintain flow at its setpoint. To work
as expected, the feedforward action would have to be on the flow
controller setpoint - multiplied by a not summed with the pH controller
output. Multiplication will force the reagent flow to zero if
the process fluid flow is zero or the fluid is at the setpoint.
Also for you control jocks, multiplication cancels composition
loop gain - a term inversely proportional to sump flow. This leads
to Fact of Life #6.
All
of these corrections are reflected in Figure 2b above. The system,
as shown, has been controlling well since startup.
Where's
the agitator?
A process used a vertical tank of neutralization. Performance
was poor because response was slow and the effluent was not uniformly
mixed. I looked at the drawings and noted that the vertical unit
seemed a bit tall for its diameter. I asked how high it was, and
the designer said, "50 feet," I gasped, "It's not nice to kid
an old engineer." He responded, "Who's kidding?" He replied, "You're
the only agitator on this project." I instantly knew I had a major
problem.
FIGURE
3. Where's the agitator? (a)-unsuccessful and (b)-successful
pH control systems for a process involving an extremely tall
mixing tank without an agitator
.
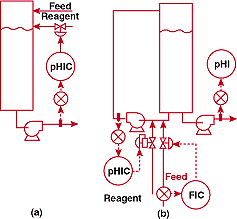
Figure 3a shows how the pH was originally being controlled. Axial
agitation probably would have corrected the difficulties, but
could not be provided economically because the tank was too tall.
A shorter tank would also have worked - again at a higher price
than the plant wanted to pay. I decided that the best way to cope
with the tank would be to use its volume as a filter, estimating
that it would attenuate the hydrogen ion concentration oscillations
of an in-line loop by a factor of 10,000 - 4 pH units. A circulation
pump was installed as a low-deadtime in-line mixer. Influent and
reagent were added to the new suction; an injector probe was installed
on the pump discharge. The new system is shown in Figure 3b.
Upsets
still occurred, due mainly to the quick opening characteristic
and the large positioner hysteresis of the plug valve on the influent.
However, the in-line pH loop returned rapidly to setpoint after
a disturbance. Further, after passing through the tank volume,
the pH drew the straightest line I have ever seen; for a moment,
we thought someone had tied down the pointer. Performance was
so good that the plant suggested we standardize on this type of
system for pH control. I warned them that the setpoint of this
system was several pH units below the neutral zone, on a relatively
flat position of the titration curve. On a steep part of the curve,
Fact of Life #2 would prevail and there would be lots of oscillations.
FIGURE
4. Where's the electrode? (a)-unsuccessful and (b)-successful
pH control systems for a process in which electrodes have
to be installed in inconvenient locations.
Where's
the electrode?
I
was called in to troubleshoot the pH system shown in Figure 4a.
This simple configuration should have worked flawlessly, but was
plagued by an unacceptably wide control band about the setpoint.
I went down to look at the exit nozzle of the vessel and couldn't
find the electrodes. I rapidly surmised that I had a major problem.
In this case, the source of the difficulty was political. The
instrument maintenance department had specified that the electrodes
be located in the analyzer house, to avoid the discomfort of servicing
them outside during the winter. Unfortunately, this location introduced
excessive deadtime in the loop. To help avoid this problem in
other situations, I feel compelled to state Fact of Life #7.
I succeeded in getting the electrodes moved by arguing about the
extreme safety hazards and product quality problems that accompanied
large pH excursions. The change, indicated in Figure 4b, narrowed
the control band to about 0.1 pH.
We
used injector electrodes for this application. Experience shows
that these provide better performance and require less maintenance
than sample chamber electrode holders. These benefits are especially
evident when the electrodes are mounted in the discharge nozzle
piping where fluid velocity is high - because the flow ensures
rapid response by minimizing boundary layer thickness and prevents
electrode coating by impurities in the stream.
Injection
electrodes also appear to be less prone than sample chamber elements
to leakage. In checking 30 installations of injection devices
from one manufacturer, I found no instances of leakage; in fairness,
when we obtained products from a different source, some leakage
did occur. However, every sample chamber electrode holder I have
ever encountered has eventually leaked. Moreover, leakage is visible
with injector assemblies but not with sample chambers. For hazardous
fluids, you don't want any surprises when you open the top cover
of the electrode holder. This leads me to Fact of Life #8.
Is
bigger better?
A
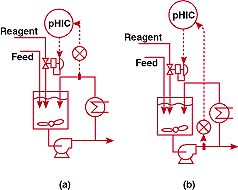
plant used the system of Figure 5a for waste neutralization. The
eductor shown in the figure had been added because mixing deadtime
was too long. But even with this device, the deadtime appeared
to be over 40 minutes. The consequent natural period of the pH
loop was 160 minutes, so the maximum reset should have been less
than 0.01 repeats per minute. Since this was below the minimum
setting on the controller, the loop was in a continuous reset
cycle; further, the integrated error - which is proportional to
the deadtime squared - was out of this world. I looked at the
engineering flow diagram and spotted the largest storage tank
I had ever seen. I asked the process engineer where the neutralization
tank was, and he pointed to the elephant I just thought was for
storage. I immediately understood that I had major problem.
The
intent of the large tank was plausible. It would serve to blend
acidic and basic waste streams from different sources and minimize
the reagent demand. Now, as long as you don't have to put control
loops on them, large tanks are useful. Upstream of a control loop,
a large tank can filter out disturbances and reduce reagent requirements;
downstream, it can filter out loop oscillations - which is particularly
advantageous because these fluctuations are usually faster than
variations in influent concentration and are therefore more effectively
attenuated. This reminds me of Fact of Life #9.
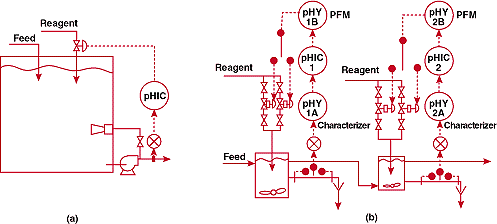
FIGURE
5. Is bigger better? (a)-unsuccessful and (b)-successful pH
control systems for a process in which an extremely large
tank was initially employed for mixing.
The
new control system is shown in Figure 5b. The large tank was replace
with two small vessels in series. A pulse frequency controller
was installed to avoid valve pluggage at low reagent flows and
to meet the extreme rangeability requirements imposed by the wide
variations in influent flow and pH. Signal characterization was
used to counteract the steep slope of the titration curve at the
setpoint.
Startups are no fun without magical mystery. In this instance,
we noticed that the pH measurement on the first tank was erratic.
The problem could not be duplicated when we removed the electrodes
and inserted them directly into the buffer solution or connected
them to the measurement system of the second tank. We replaced
the pH transmitter, preamplifier, cable, and electrodes individually
but the erratic measurements continued. Eventually, someone remembered
that the fiberglass preamplifier enclosure supplied by the manufacturer
was replaced by the field maintenance department with a metal
housing - to provide more room for access. The enclosure mounting
plate was grounded. This created a second ground point in the
circuit, and caused a significant current flow through the circuit.
The problem did not occur on the second tank because the preamplifier
housing was not mounted on a conductive structure. Likewise, the
erratic behavior was not observed during buffering because the
bottle was plastic. The problem was solved by isolating the preamplifier
enclosure from ground with a plastic mounting plate.
The control system has performed well from startup except for
periodic pluggage of the electrodes in an overflow sample line.
Liquid head is too low to achieve a sample velocity sufficient
to sweep the electrodes clean. A new electrode holder that provides
a large flat electrode surface will be tried. If that doesn't
work, we may have to shake loose enough money to install a sample
pump and an injector electrode assembly.
Where's
the reagent piping?
The pH in a neutralization tank was fluctuating in what appeared
to be a square wave. The system was also subject to periodic glass
electrode failures caused by etching and severe upsets due to
a high-temperature interlock that sets due to a high-temperature
interlock that shut off the reagent flow. Plant people were especially
anxious to improve this system because reliability was critical
to plant productivity. I stood at the top of the vessel wondering
what to do, and noticed that the reagent was being transported
by a conveyor rather than a pipe. I soon perceived that I had
a major problem.
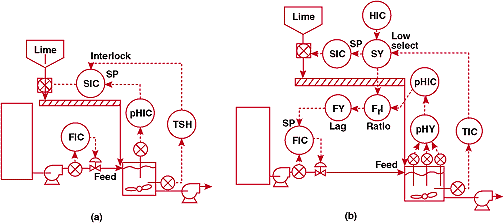
FIGURE
6. Where's the reagent piping? (a)-unsuccessful and (b)-successful
pH control systems for a process iin which a powdered line
reagent is delivered by a conveyor.
Figure
6a shows the original installation. The reagent, pulverized lime,
was controlled by a rotary feeder at the discharge of the hopper.
Feeder speed was set by the pH controller output. Reagent delivery
was subject to several minute's lag due to transportation delay
on the conveyor and solids dissolution time. We made precise measurements
of the pH in the tank and found that the square waves were worse
than the plant thought - the process instruments recorded only
the high end of the pH scale, but the fluctuations actually covered
almost the whole range from 0 to 14.
Luckily,
a huge tank upstream of the waste flow provided enough inventory
so the pH controller could be used to throttle the waste stream.
The lime feeder speed was determined by selecting the lower of
a manually-entered throughput setpoint and a command from the
temperature override controller. The low signal selector therefore
provided smooth transition between between normal and override
control. The feeder speed signal is also multiplied by the pH
controller command, passed through a lag unit whose delay is set
equal to the reagent delivery time, and fed forward to establish
the waste flow setpoint.
To
eliminate downtime due to electrode failures, a system was installed
using three measuring elements and voting logic to establish the
output signal. Use of three rather than two electrode assemblies
make it possible to determine which signal to use, if the electrode
outputs disagree. This leads me to Fact of Life #10.
Control
improved dramatically. Electrode failure due to etching, which
had occurred when the solution was acidic - at the unrecorded
lower portion of the square wave - also stopped. And use of voting
logic to control using three electrode assemblies has virtually
eliminated downtime, even when an element becomes nonfunctional.
USING
YOUR SKILLS
One of the prices you pay for being an instrumentation expert
in the processing industries is that occasionally, someone will
ask you to control pH. The job rarely proves to be easy, for instance
because you are on a flat portion of the titration curve or have
wide tolerance on response and accuracy, because changes are then
high that someone has done it satisfactorily without you. So the
problems you get are usually major problems. You'll have to call
on all you know about the installation and operation of electrodes,
control valves, piping, and mixing equipment. You'll have to brush
the cobwebs off your basic understanding of feedback and feedforward
loop strategies. You'll have to home your skills as a diplomat
to get the plant to install, replace, or eliminate vessels or
instruments that make life convenient for the operators or maintenance
people - or represent investments for which somebody has a neck
on the line - but are preventing satisfactory pH control. And
you'll have to resign yourself to living out of your suitcase
for a while while the plant starts up and experiences the magical
mystery of pH.
REFERENCE
1.
McMillian. G K; pH Control. Independent Learning Module Series,
Instrument Society of America (Research Triangle Park NC), 1964.
©
Instruments Society of America. Intech, September, 1984. All rights
reserved.
| 






