PFA Fluorocarbon Information
PFA Fluorocarbon Resin
Table
1
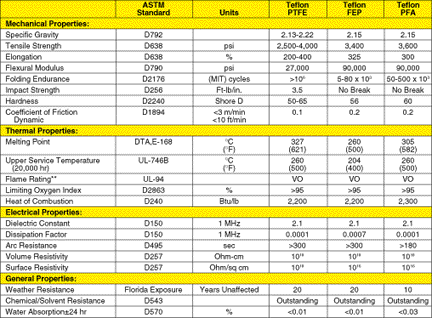
*Typical values are unsuitable for specifications. Properties were measured
at 23 degreesC (73 degreesF), unless otherwise noted.
**Statements regarding behavior in a flame situation are not
intended to reflect hazards presented by this or any other material when under actual fire conditions.
The following characteristics contribute to the unique properties of PFA fluorocarbon resins:
• Nonpolarity:The carbon backbone of the linear
polymer is completely sheathed by the electron cloud of fluorine atoms, much like a wire core
is protected by insulation coating. This ensheathment, and the angles at which the carbon-fluorine
bonds are disposed, causes the centers of electronegativity and electropositivity to be
perfectly balanced across the polymer chain cross section. As a result, no net charge difference
prevails. This nonpolarity of the polymer is partly responsible for its lack of chemical
reactivity.
•Low interchain forces: The bond forces between two
adjacent polymer chains are significantly lower than the forces within one chain.
PFA PTFE linear polymer chains are otherwise restrained. However, in PFA FEP and PFA,
interpolymer chain entanglement of the pendant structure precludes the shifting of polymer
chains to relieve the implied load. The "creep" normally associated with PFA PTFE is mostly
avoided with PFA FEP and even more so with PFA.
• High C-F and C-C bond strengths are among the strongest
in single bond organic chemistry. The polymer must absorb considerable energy to
disrupt these bonds. Chemical reactions represent a kinetic and thermodynamic
resolution of bond-making and bond-breaking in favor of the most stable system.
These bond strengths are hard to overcome.
• Crystallinity:The high degree of crystallinity in
these semicrystalline polymers results in high melting points, mechanical properties, and an
integral barrier to migrating, small, nonpolar molecules. Under certain conditions, these molecules
penetrate the plastics.
•High degree of polymerization: The unbranched nature
of the polymers and their low interpolymer chain attraction requires very long chain lengths
in PFA PTFE and entanglement in PFA FEP and PFA to provide load-bearing mechanical properties.
The chain length also has an impact on flow and crystallinity of the polymers. These unique
properties lead to the following benefits:
•High melting points (327°C [621°F] for PFA PTFE; 260°C [500°F] for
PFA FEP, and 305°C [582°F] for PFA PFA). The melting point of PFA PTFE is one of the highest
in organic polymer chemistry. Other materials can attain higher temperatures, but they degrade
rather than melt. Compared to PFA PTFE, the lower melting temperature of PFA FEP results from
lower°of polymerization and crystallinity. In PFA PFA, a higher degree of polymerization,
enhanced entanglement of the pendant structure, and lower comonomer content combine
to provide a melting point closer to that of PFA PTFE.
•High thermal stability: Due to the strength of the
carbon-fluorine and carbon-carbon single bonds, appreciable thermal energy must
be absorbed by the polymers before thermal degradation. The rate of decomposition of
a part of PFA depends on the particular resin, temperature, and heat exposure time; and to
a lesser extent, pressure and nature of the environment. At maximum continuous
service temperatures, thermal degradation of the resins is minimal. For example, at 400°C,
PFA FEP is measured at 4/100,000 of 1 percent, and PFA PTFE at 1/100,000 of 1 percent.
At high processing temperatures, adequate ventilation is recommended.
• High upper service temperature (260°C [500°F] for PFA PTFE, 204°C
[400°F] for PFA FEP and 260°C [500°F] for PFA). The polymers' high melting points and
morphological features allow components made from the resin to be used continuously at the stated
temperatures. Above this temperature, the component's physical properties may begin to decrease.
The polymer itself, however, will be unaffected if the temperature is insufficient for thermal degradation.
•Insolubility: There is no known solvent for PFA fluorocarbon
resins under ordinary conditions.
• Inertness to chemical attack:The intrapolymer-chain
bond strengths preclude reaction with most chemicals. Under relatively unusual circumstances
the polymer can be made to react. Examples of unusual reagents include:
- Sodium, in a suitable media, etches the fluorocarbon polymer.
- Finely divided metals often interact with the polymer.
- Interhalogen compounds often induce halogen interchange with the fluorine.
- Ionized oxygen in oxygen plasma is often sufficiently energetic
to react with the polymer chain.
- Electron bombardment at the megarad level can sever the polymer
chain.
• Low coefficient of friction: The low coefficient of friction of
PFA results from low interfacial forces between its surface and another material and the comparatively
low force to deform.
•Low dielectric constant and dissipation factor: PFA
provides low, if not the lowest, values for these parameters. These low values arise from the
polymer's nonpolarity as well as the tight electron hold in the ultrapolymer bonds.
•Low water absorptivity: For PFA to absorb water,
the surface must remain wet for a long enough time for water to become physico-chemically associated
with the polymer chains, and then it must become included in the polymer bulk structure. Water is
a very high energy material and PFA has a very low surface energy. Therefore, these events are
energetically incompatible and only occur under special circumstances and to a small extent.
•
Excellent weatherability: Weather includes light of various wavelengths
(IR, visible, UV), water (liquid or gas), other gases, and normal
temperatures and pressure. The physical and chemical makeup of
PFA makes it inert to these influences.
•Flame resistant:PFA will burn when exposed to flame,
but will not continue to burn when the flame is removed.
•Excellent toughness: Some mechanical properties of PFA resins are
shown in Table 1. Toughness characteristics are high and differ somewhat between resin types.
Tefzel Fluoropolymer Resin
Replacement of fluorine in fluorocarbon polymers is only commercially
successful when the fluorine is replaced by hydrogen or hydrogen
and chlorine. However, the resulting polymers have significantly
different properties from those of fully fluorinated resins. When
this substitution occurs by regular alternation, polarity and
mechanical properties are maximized. The polymer's polarity increases
because the substituting elements:hydrogen and chlorine:have different
electronegativities relative to fluorine. Also, the length of
their bonds to carbon of the polymer backbone differ. Thus, the
centers of electronegativity and electropositivity are not balanced
between chains. The increased interpolymer chain attraction results
in higher mechanical properties. In addition, the increased polarity/interpolymer
attraction influences penetrants' permeation of the resin's amorphous
component.
However, the presence of hydrogen or of hydrogen and chlorine sacrifices
chemical and thermal stability. For example, in simple molecules,
the C-H bond is ~5 percent weaker than the C-F bond, and the C-C1
bond is 25 percent weaker.
In addition to weak chemical bonds, the arrangement of the substituting
elements along the polymer chain has a marked effect on the resin's
chemical stability. In this regard, solubility can be a leading
indicator. Tefzel, with a regularly alternating structure of the
monomers tetrafluoroethylene and ethylene, has no known solvent
in ordinary conditions. In contrast, polyvinylidene fluoride,
the chemical isomer of Tefzel, is soluble in common industrial
ketones (e.g., methyl ethyl ketone). Ethylene/trifluoroethylene
is soluble in some fluorinated solvents. The substituted polymers
are also adversely affected by strong acids and alkalies. Of the
three mentioned, Tefzel is compatible with the broadest range
of chemicals under a wide range of conditions.
Equal proportions of the comonomers react to produce a polymer where
individual monomers alternate regularly along its chain.
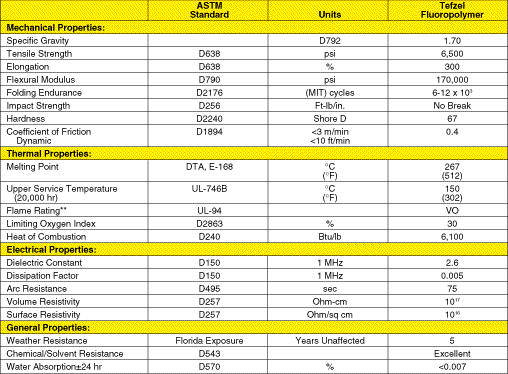
Typical properties of Tefzel appear in Table 2. Data in Table 2 and Table
1 show that the polarity and accompanying interpolymer chain attraction
enhance the physical properties of the substituted polymer over
those of the unsubstituted, fully fluorinated polymer. Note, for
example, that Tefzel has about 1.5 times greater strength than
PFA and 2 times greater stiffness.
Tefzel Fluoropolymer Resin Table 2
Summary and Conclusion
The chemistry and physicochemistry
inherent in the fully fluorinated polymer structures allow PFA FEP and PFA fluorocarbon resins to
provide unique resin component benefits for chemical corrosion resistance. Tefzel fluoropolymer
resin comes closer to PFA than any other partially fluorinated resin, in chemical and electrical
properties, while providing enhanced mechanical ruggedness and economical processing.
|

